StemInov
À propos de votre organisation / profil
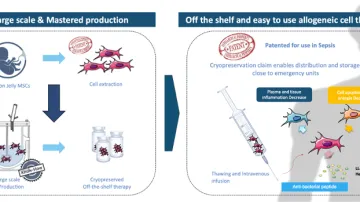
•StemInov is developping WhartSep® a stem cell based therapy which has a unique, 3 in 1, adaptive mode of action to treat septic shock & ARDS (patented):
- Ability to migrate to damaged organs and tropism for lungs
- Direct and indirect antibacterial effect
- Pro- or anti-inflammatory action, depending on the environment (modulation of inflammation)
•WJ-MSC have already been tested in human (Phase I/II in COVID 19) and showed to be strongly effective in reducing ventilation time and shortening hospital stay
•WhartSep® is an allogeneic off-the-shelf and ready to use WJ-MSC cell therapy that fits large diffusion needs thanks to its capacity to be stored frozen. Moreover, our unique know-how in manufacturing endowed the company with an inexhaustible production process ready to fulfill the need for large-scale production: raw material is easily accessible, batches are highly reproducible with cell properties preserved and homogeneous, production costs are dropped.
•>400 clinical trials with WJ-MSC: safety admitted and great promise for collaboration on our platform
StemInov targets to develop Whartsep® up to Phase IIa of clinical trials. This stage will give the company the opportunity to license/sell Whartsep® to a Pharma partner and generate revenues through upfront payments, milestones payments, and royalties. In parallel, thanks to its platform, StemInov will also lead collaborative programs with industry / academia in other indications using WJ-MSC (auto-immune diseases, brain & neuro disorders, eye diseases, regenerative medicine, heart and cardiovascular)